67-Year-Old Man With ALL, Febrile Neutropenia, and a Lower Respiratory Tract Infection

Presented by Dr Joy Sarojini Michael
*Image is representative and is not of the actual patient.
Case Presentation
A 67-year-old man recently diagnosed with B-cell acute lymphoblastic lymphoma (ALL) was started on chemotherapy. On day 10 of chemotherapy, he developed a fever of 101oF (38.3oC) and a total white blood cell count of 300 cells/mm3 with neutropenia (0% neutrophils).
He developed oral candidiasis, and blood cultures were positive for Pseudomonas aeruginosa. He was started on intravenous (IV) ceftazidime for 14 days and subsequently developed tachypnoea and respiratory distress. On examination, he had bilateral crepitations. He was taken to intensive care and mechanically ventilated.
A high-resolution computed tomography (CT) scan of the thorax was carried out (see Figure).
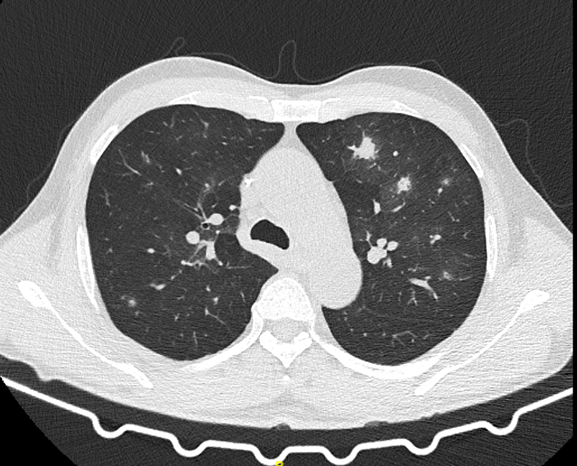
Figure. CT of the thorax of the patient. Image courtesy of Dr Joy Sarojini Michael.