Discussion:
Correct answer: 1
Guidelines for management of mucormycosis are based on consensus recommendations.8,9 Amphotericin B and amphotericin B-based lipid formulations remain the agents of choice for empiric management of mucormycosis.8,9 However, amphotericin B has notorious toxicities and requires IV administration. While posaconazole and isavuconazole are available orally with fewer toxicities, their in vitro activity against the Mucorales is not predictable.10,11 While there are no established clinical breakpoints for the Mucorales for any of the antifungal drugs, standardized susceptibility testing to isavuconazole and posaconazole could provide insight regarding the level of activity for each drug against this patient’s specific pathogen, thereby guiding choice of step-down therapy.
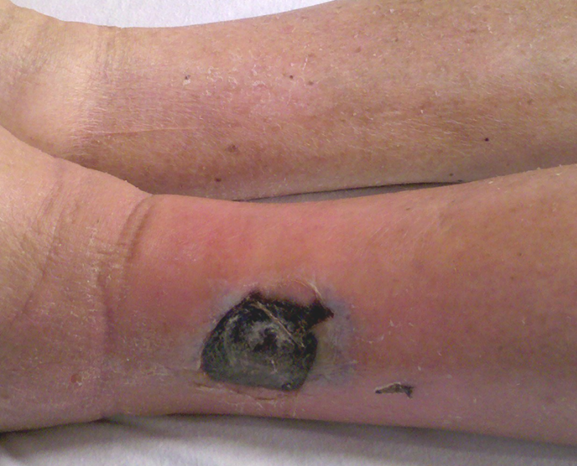
Diagnosis of mucormycosis is typically made based on a combination of clinical, imaging, culture, and histopathologic findings.
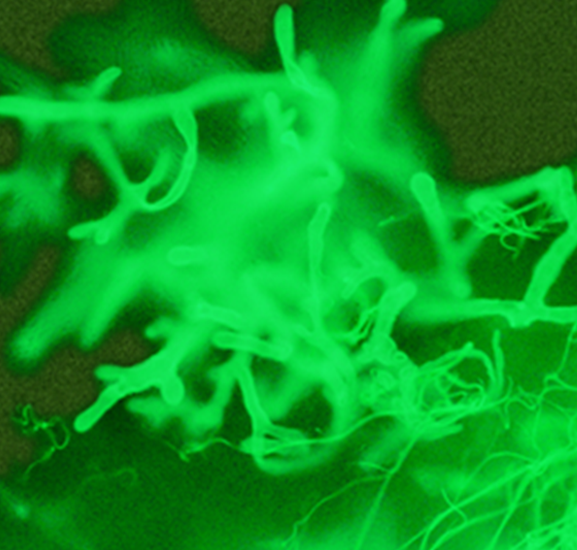
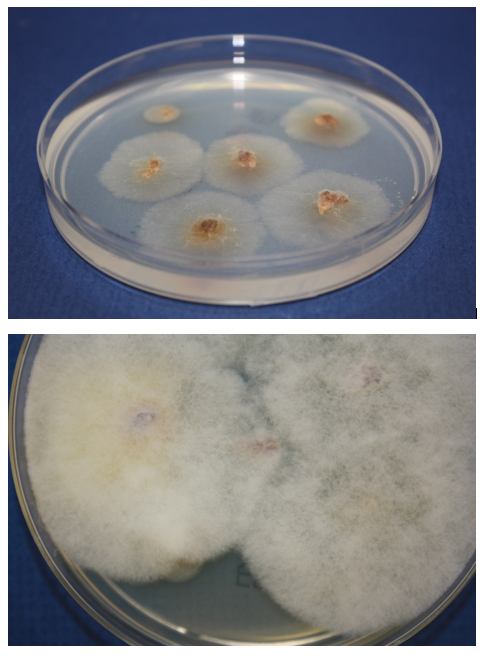
Grinding tissue for culture risks disrupting the fragile and sparsely septate hyphae, resulting in no viable cells. Instead, tissue should be finely minced and placed directly on fungal media.4 When recovered, these fungi grow rapidly on laboratory media, producing abundant cottony colonies (Figure 3b). Identification is based on their sporangial structures.8 However, culture is only approximately 50% sensitive for their recovery, even in microscopically positive samples. Thus, molecular-based assays for detection of Mucorales DNA in culture-negative specimens are emerging as a key means of identifying the pathogen, particularly when the organism does not grow in culture.8
Answers 2 and 3 are incorrect. In the current case, the fungal pathogen was recovered in culture, thus extraction of DNA and sequencing for identification would add unnecessary expense. Identification of Rhizopus to species level also does not offer further information to guide management.
Answer 4 is incorrect. The non-invasive blood-based beta-D-glucan and galactomannan tests are not helpful in the diagnosis of mucormycosis because Mucorales species lack significant quantities of these polysaccharides in their cell walls.9
There is growing interest in the utility of molecular testing to noninvasively detect Mucorales DNA, but such testing is not approved by the United States Food and Drug Administration at this time. One prospective trial found the sensitivity and specificity of a serum Mucorales quantitative PCR to be 85.2% and 89.8%, respectively, and the first positive PCR was seen a median of 4 days (interquartile range 0-9 days) before the first positive mycological or histologic specimen in their study.12 Another study retrospectively analyzed archived specimens from hematopoietic stem recipients with invasive pulmonary mold (primarily Aspergillus and Mucorales) infection for the presence of microbial cell-free (mcf) DNA in plasma (Karius Laboratory, Redwood City, CA). The median mcfDNA concentrations were higher in Mucorales compared with Aspergillus cases, and high mcfDNA concentrations at diagnosis (mcfDNA concentrations >4.0 log10 molecules per microliter) were associated with 6-week mortality.13,14 Left to be explained is the variation in fungal DNA concentrations for Aspergillus compared with Mucorales infections. Ultimately, further study is needed to understand the role of molecular testing for diagnosis of Mucorales infections as well as the implications of fungal DNA concentration and kinetics on therapeutic response.