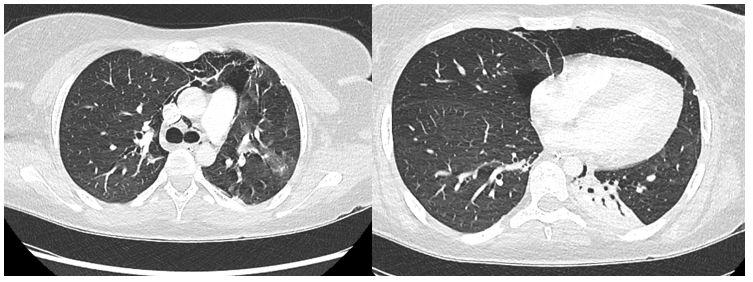
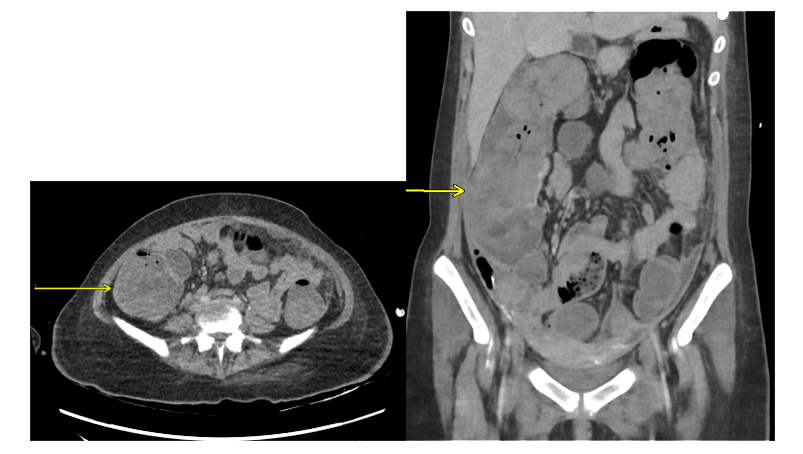
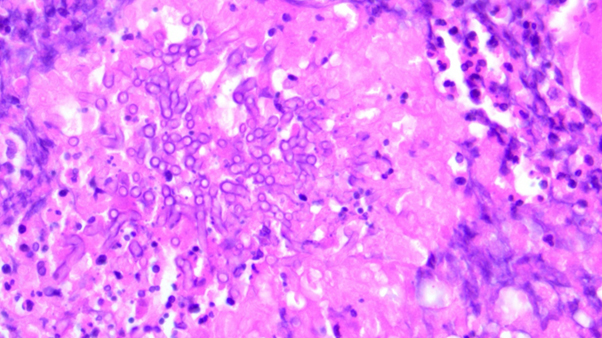
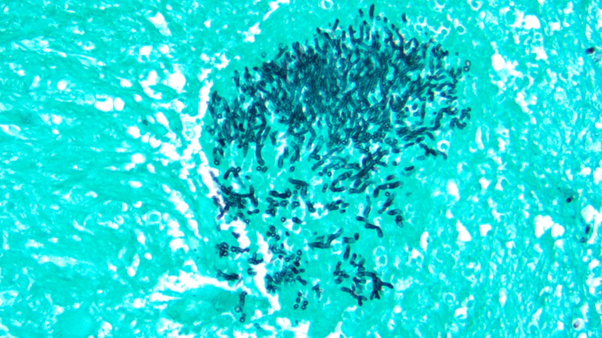
15-Year-Old Female Presents to the Emergency Department in Septic Shock
Presented by Dr Alida Fe Talento
*Image is representative and is not of the actual patient.
Case Presentation
A previously well 15-year-old female presented to the emergency department (ED) with symptoms of fever, vomiting, diarrhoea, and lethargy. These symptoms were preceded by a 2-week history of an upper respiratory tract infection. On examination at the ED, she was tachycardic, hypotensive, tachypnic, and was noted to have cold, clammy, and poorly perfused extremities. The patient reported decreased urine output. Abdomen was soft and non-tender. On gynaecological examination, she was noted to have foul-smelling, green discharge per vagina. The patient was resuscitated in the ED and transferred to the paediatric ICU (PICU) for further management with a preliminary diagnosis of septic shock. She was started on broad-spectrum antimicrobial therapy (meropenem, clindamycin, vancomycin and acyclovir) after a full septic screen. She received supportive treatment in PICU, which included mechanical ventilation, renal replacement therapy, and inotropic support.
Preliminary laboratory results showed metabolic acidosis, lactic acidosis, leucocytosis with neutrophilic predominance, low haemoglobin, thrombocytopenia, and coagulopathy. Inflammatory markers, including procalcitonin, C-reactive protein, and interleukin-6, were markedly elevated. Methicillin-susceptible S. aureus was isolated from vaginal swabs. Low levels of SARS CoV-2 virus were detected on a polymerase chain reaction (PCR) test of a nasopharyngeal swab. Blood cultures were sterile. The diagnosis at this point was toxic shock syndrome due to S. aureus vs PIMS-TS (paediatric inflammatory multi-systemic syndrome following SARS-CoV-2 infection). Antimicrobial therapy was changed based on the culture results.
With supportive treatment, there were initial signs of response wherein inotropic and ventilatory support were slowly weaned. She continued to have high-grade fever and was persistently thrombocytopenic, with low haemoglobin and deranged bleeding parameters. Ferritin, fibrinogen, and creatinine kinase levels were all markedly elevated. Renal replacement therapy was resumed. Due to her persistent symptoms and laboratory results, which suggested an ongoing inflammatory process, she was referred to the Haematology Service. On her 10th hospital day, a bone marrow aspiration was performed, which revealed findings consistent with haemophagocytic lymphohistiocytosis (HLH) most likely due to the preceding S. aureus infection. Due to the above findings, she was prescribed intravenous (IV) immunoglobulin, IV pulsed methylprednisolone, and anakinra.